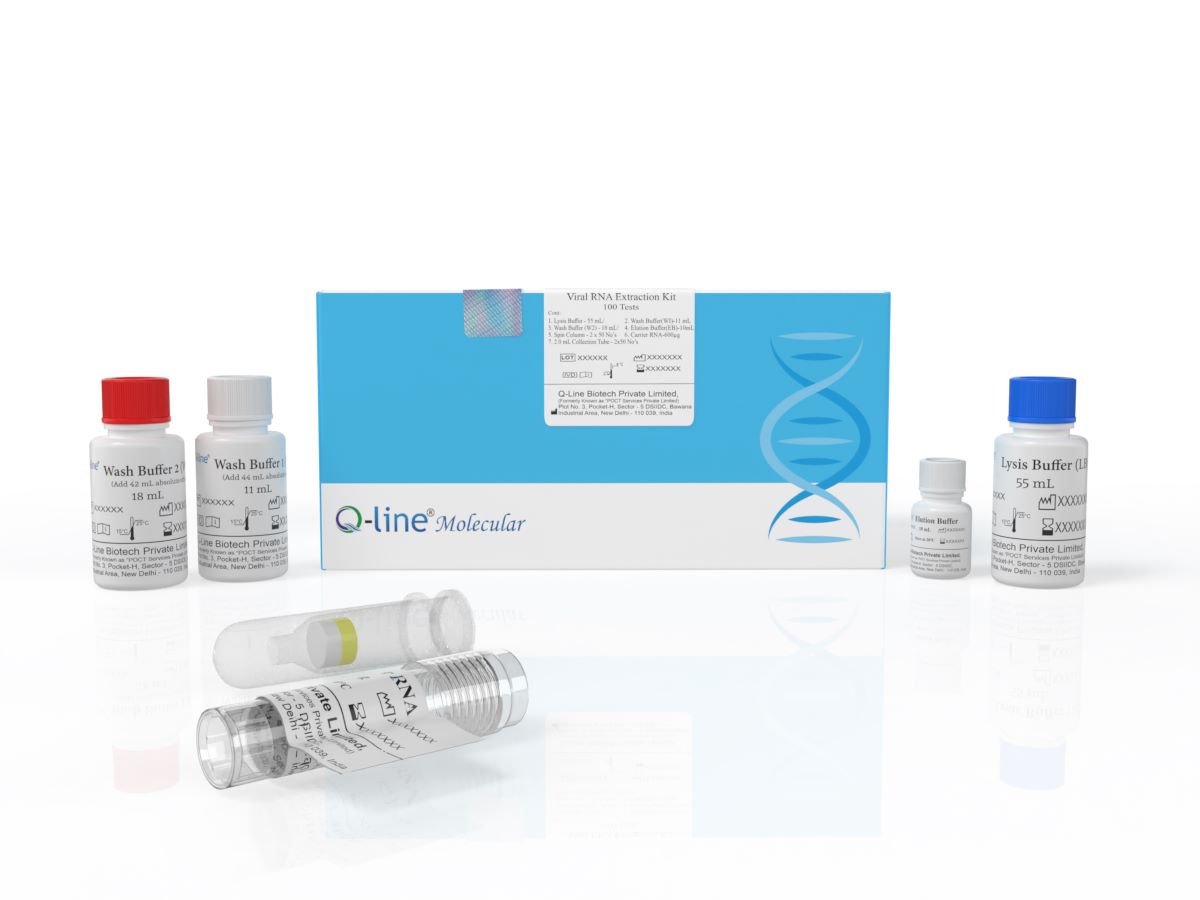
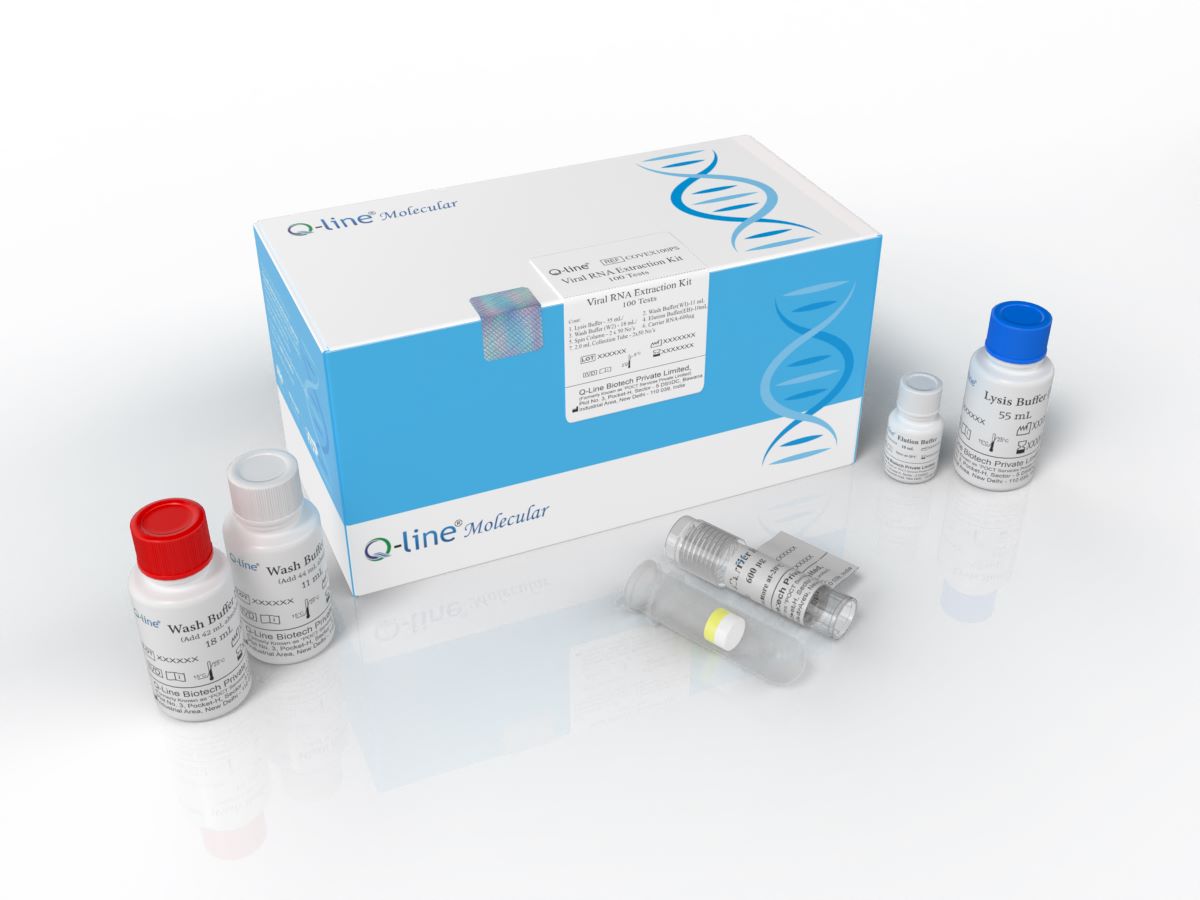
Viral RNA Extraction Kit (Manual Method)
Product Description
Manual Magnetic Bead Method
Available Options:
| REF | Volume |
|---|---|
| MDVRXM0050QB | 1 x 50 Test |
| MDVRXM0100QB | 1 x 100 Test |
Q-Line® Molecular Viral
RNA Extraction Kit (Magnetic Bead Manual) is an advanced solution designed for
the efficient isolation of high-quality RNA from viral samples such as blood,
plasma, serum, saliva, swabs and other body fluid. Utilizing state-of-the-art
magnetic bead technology, this kit offers unparalleled performance, ensuring
reliable and consistent RNA extraction for a wide range of applications in
molecular biology, virology, and diagnostics.
Key Feature:
¶
The kit provides high
yields of purified RNA, ensuring ample material for downstream molecular
applications.
¶
It ensures high purity of
extracted RNA, free from contaminants that could interfere with downstream
analyses.
¶
The kit is compatible with
various sample types, including blood, plasma, serum, saliva, swabs and other
body fluid making it suitable for a wide range of applications.
¶ With a streamlined protocol and optimized reagents, the kit enables fast and efficient extraction of viral RNA, saving time in the laboratory.
¶
The kit undergoes stringent
quality control measures and validation to ensure consistent and reliable
performance, minimizing variability between extractions.
¶
It is compatible with
manual method, providing flexibility to suit different laboratory setups and
throughput requirements.
¶
The kit is user-friendly,
with clear protocols and convenient reagent formats, making it accessible to
both experienced researchers and novices in molecular biology.
¶
It is suitable for a wide
range of applications, including PCR, RT-PCR, sequencing, and other molecular
biology techniques, making it invaluable for research and clinical diagnostics
in virology and infectious diseases.
 English
English
 French
French
 Spanish
Spanish
 Russian
Russian
 Arabic
Arabic